Rheumatoid Arthritis Patients, Both Newly Diagnosed and Methotrexate Treated, Show More DNA Methylation Differences in CD4+ Memory Than in CD4+ Naïve T Cells
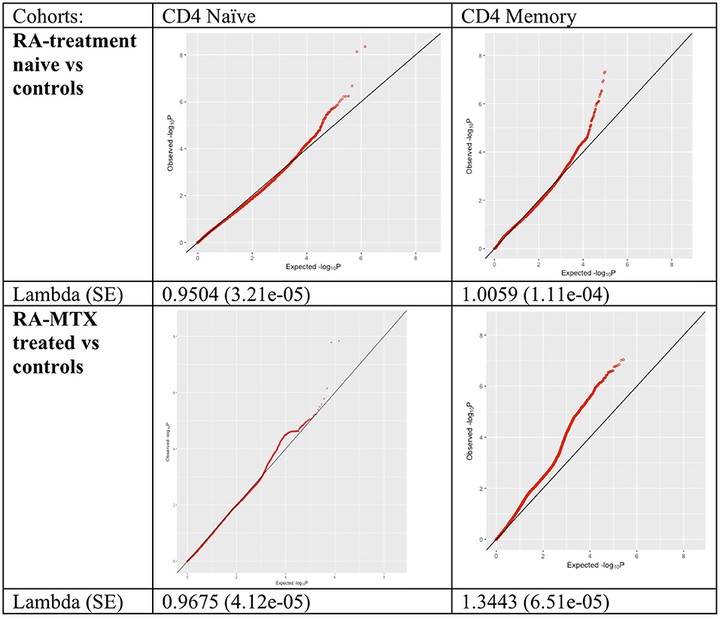 Image credit: Unsplash
Image credit: Unsplash
Abstract
Differences in DNA methylation have been reported in B and T lymphocyte populations, including CD4+ T cells, isolated from rheumatoid arthritis (RA) patients when compared to healthy controls. CD4+ T cells are a heterogeneous cell type with subpopulations displaying distinct DNA methylation patterns. In this study, we investigated DNA methylation using reduced representation bisulfite sequencing in two CD4+ T cell populations (CD4+ memory and naïve cells) in three groups - newly diagnosed, disease modifying antirheumatic drugs (DMARD) naïve RA patients (N = 11), methotrexate (MTX) treated RA patients (N = 18), and healthy controls (N = 9) matched for age, gender and smoking status. Analyses of these data revealed significantly more differentially methylated positions (DMPs) in CD4+ memory than in CD4+ naïve T cells (904 vs. 19 DMPs) in RA patients compared to controls. The majority of DMPs (72%) identified in newly diagnosed and DMARD naïve RA patients with active disease showed increased DNA methylation (39 DMPs), whereas most DMPs (80%) identified in the MTX treated RA patients in remission displayed decreased DNA methylation (694 DMPs). Interestingly, we also found that about one third of the 101 known RA risk loci overlapped (±500 kb) with the DMPs. Notably, introns of the UBASH3A gene harbor both the lead RA risk SNP and two DMPs in CD4+ memory T cells. Our results suggest that RA associated DNA methylation differences vary between the two T cell subsets, but are also influenced by RA characteristics such as disease activity, disease duration and/or MTX treatment.